I.6. Proprietăți fizice și chimice.
- I.6.1. Proprietăţi fizice.
- I.6.2. Proprietăţi chimice.
- I.6.3. Observarea proprietăților fizice și chimice ale unor substanțe.
- I.6.4. Aplică ce ai învăţat la Proprietăţi fizice şi chimice.
Însușirile caracteristice cu ajutorul cărora se recunoaște o substanță se numesc proprietăți.
Proprietăţile pot fi fizice și chimice.
I.6.1. Proprietăţi fizice.
Proprietăţile fizice sunt acele însușiri care se referă la aspectul sau la transformări care nu schimbă compoziția substanței.
Proprietăţile fizice se pot clasifica în:
1. Observabile cu ajutorul organelor de simț:
- Prin văz: starea de agregare (solidă, lichidă, gazoasă), culoarea (incolor-fără culoare sau colorat), luciu (strălucirea).
- Prin miros: inodor (nu are miros), plăcut, iritant, miros specific (laptele, clorul, oțetul - spunem că au miros specific).
- Prin pipăit: plasticitate, elasticitate, moale (duritate), tare (duritate).
2. Măsurabile cu ajutorul unor aparate:
- Constante fizice:
- Temperatura de topire (temperatura la care începe să se topească o substanță solidă=Tt);
- Temperatura de fierbere (temperatura la care începe să fiarbă un lichid = Tv);
- Densitatea

- Indicele de refracție
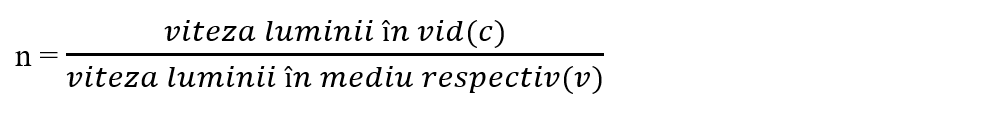
- Coeficient de solubilitate (arată ce cantitate de substanță se dizolvă în 100 g apă, la o anumită temperatură);
- Duritatea (se măsoară după o scară de duritate de la 1 la 10, numită „Scala lui Mohs”, în care talcul are 1 și diamantul cea mai mare duritate 10);
- Conductibilitatea electrică și termică (trecerea curentului electric, respectiv a căldurii, fără deplasare de substanță).
I.6.2. Proprietăţi chimice.
Proprietăţile chimice sunt acele însușiri care se referă la transformări care schimbă compoziția substanței.
Exemple de proprietăţi chimice:
- Proprietatea de a arde (după arderea hârtiei, nu mai avem hârtie, avem cenușă);
- Proprietatea grăsimilor de a râncezi (râncezeala este o altă substanță decât uleiul nerâncezit);
- Proprietatea vinului de a se oțeti (oțetul este altă substanță decât vinul);
- Proprietatea laptelui de a se acri (laptele acru are altă compoziție și alte proprietăți decât laptele dulce);
- Proprietatea fierului de a rugini (rugina este altă substanță decât fierul);
- Proprietatea cuprului de a cocli (cocleala este o substanță diferită de cupru;
- Proprietatea lemnului de a putrezi (putregaiul are altă compoziție decât lemnul).
I.6.3. Observarea proprietăților fizice și chimice ale unor substanțe.
👀 Experiment: Proprietatea fierului de a rugini
Materiale necesare:
Eprubetă, pilitură de fier, vas cu apă.
Descrierea experimentului:
- Umezește o eprubetă și pune pe fundul ei pilitură de fier și răstoarn-o cu gura în jos într-un vas cu apă.
- Așteaptă o zi și vezi ce se întâmplă.
Concluzia experimentului:
Fierul are proprietatea de a rugini - proprietate chimică, fiindcă fierul își schimbă compoziția și se transformă în
rugină.
Proprietatea unor substanțe de a arde
👀 Experiment: Arderea magneziului cu o flacără orbitoare
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
🔥 Atenție! A nu se privi flacăra orbitoare de la arderea magneziului întrucât aceasta produce leziuni ale ochilor!
Materiale necesare:
Magneziu panglică, spirtieră, clește metalic, chibrit.
Descrierea experimentului:
- Se ține cu cleștele o panglică de magneziu în vârful flăcării unei spirtiere și se observă că după un timp, magneziu se aprinde și arde cu o flacără orbitoare și se transformă într-un praf alb.
Concluzia experimentului:
Unele substanțe au proprietatea de a arde - proprietate chimică, întrucât substanța și-a schimbat compoziția (magneziul
s-a transformat în oxid de magneziu).
Proprietatea unor substanțe de a arde
👀 Experiment: Arderea cuprului
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
Materiale necesare:
Sârmă de cupru, spirtieră, clește metalic, chibrit.
Descrierea experimentului:
- Se ține cu cleștele o sârmă de cupru în vârful flăcării unei spirtiere și se observă că sârma de cupru arde cu o flacără verzuie și se înnegrește după ardere (se formează oxidul de cupru).
Concluzia experimentului:
Unele substanțe au proprietatea de a arde - proprietate chimică, întrucât substanța și-a schimbat compoziția.
Proprietatea unor substanțe de a arde
👀 Experiment: Arderea fierului
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
🔥 Atenție! Pilitura de fier este inflamabilă! Ai grijă să nu te arzi de la așchiile incandescente!
Materiale necesare:
Pilitură de fier, spirtieră, clește metalic, chibrit.
Descrierea experimentului:
- Presară în flacăra unei spirtiere pilitură de fier. Pilitura de fier arde cu scântei strălucitoare și se transformă într-o pulbere neagră (oxid de fier).
Concluzia experimentului:
Unele substanțe au proprietatea de a arde - proprietate chimică, întrucât substanța și-a schimbat compoziția.
👀 Experiment: Reacția dintre fier și sulfatul de cupru
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Atenție! Piatra vânătă este o substanță toxică, nu o atinge sau gusta!
Materiale necesare:
Un cui de fier, un pahar Berzelius, soluție de piatră vânătă.
Descrierea experimentului:
- Se introduce cuiul în paharul cu soluție de piatră vânătă, astfel încât o parte a sa să rămână în aer. După puțin timp se observă că partea cuiului din soluție s-a acoperit cu o substanță arămie (cupru).
Concluzia experimentului:
Fierul are proprietatea chimică de a reacționa cu sulfatul de cupru II cu formare de cupru și sulfat de fier II.
Conductibilitatea electrică a unor substanțe
👀 Experiment: Conductoare și izolatoare electrice
Materiale necesare:
Circuit format dintr-o baterie, fire de legătură, bec, sârme metalice, mină de grafit, lemn, sticlă, gumă de cauciuc.
Descrierea experimentului:
- Leagă becul la baterie și la capetele două capete libere ale circuitului se intercalează diferite substanțe (fier, cupru, aur, argint, zinc, plumb, aluminiu, grafit, sticlă, lemn, cauciuc) și observă la care substanțe becul se aprinde.
Concluzia experimentului:
Unele substanțe au proprietatea de a fi conductoare electrice, lăsând să treacă un curent electric prin ele (toate
metalele, grafitul). Această proprietate se numește conductibilitate electrică – proprietate fizică fiindcă nu schimbă
compoziția substanței. Alte substanțe sunt izolatoare electrice - adică nu lasă să treacă un curent prin ele (sticla,
lemnul, cauciucul etc.).
Conductibilitatea termică a unor metale
👀 Experiment: Ce este conducția termică
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
Materiale necesare:
Lumânare, spirtieră, clește metalic, eprubetă, apă, sârmă de cupru, chibrit.
Descrierea experimentului:
- Aprinde o lumânare și prelinge ceara topită de-a lungul sârmei, lasă să se întărească bobițele de ceară.
- Ține cu cleștele sârma ceruită în flacăra spirtierei și observă cum se topesc bobițele de ceară.
Concluzia experimentului:
Metalele au proprietatea de a conduce căldura prin ele de la capătul încălzit spre cel neîncălzit, numită
conductibilitate termică, adică sunt conductoare termice – proprietate fizică fiindcă nu schimbă compoziția substanței.
👀 Experiment: Apa este izolatoare termică
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
🔥 Atenție! Când lucrezi cu apă caldă să nu te arzi!
Materiale necesare:
Lumânare, spirtieră, clește metalic, eprubetă, apă, chibrit.
Descrierea experimentului:
- Pune într-o eprubetă apă, până la jumătatea eprubetei;
- La flacăra unei spirtiere, încălzește apa din eprubeta la partea superioară, ținând eprubeta cu mâna de partea de jos şi îndreptată cu gura în partea opusă a ta.
Concluzia experimentului:
- După puţin timp, apa de la suprafață începe să fiarbă, în timp ce în partea de jos nu se simte deloc căldura.
- Apa este rău conductoare de căldură. Toate lichidele (cu excepția mercurului, care este metal) sunt izolatoare termice. Încălzirea uniformă a lichidelor şi gazelor are loc prin convecție, cu ajutorul curenților (deplasare de substanţă).
👀 Experiment: Aerul este izolator termic
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
🔥 Atenție! Când lucrezi cu apă caldă să nu te arzi!
Materiale necesare:
Lumânare, spirtieră, clește metalic, eprubetă, chibrit.
Descrierea experimentului:
- Ia eprubeta goală (adică plină cu aer) şi introdu la capătul deschis al eprubetei un deget al mâinii;
- Încălzește fundul eprubetei (partea închisă) în flacăra spirtierei, agitând-o continuu pentru a nu se sparge.
Concluzia experimentului:
- Degetul din eprubetă nu simte deloc căldura.
- Aerul este rău conductor de căldură. În general, toate gazele sunt izolatoare termice, dar mai ales gazele rarefiate. Fibrele hainelor de lână, blana animalelor, penele păsărilor, zăpada sunt izolatoare termice, deoarece conțin aer.
👀 Experiment: Apa are proprietatea de a fierbe la 100 °C
🔥 Atenție! Acest experiment se efectuează numai în prezența unui adult!
🔥 Atenție! Când lucrezi cu surse de foc ai grijă să ai părul strâns și să nu porți haine cu mâneci largi!
🔥 Atenție! Când lucrezi cu apă caldă să nu te arzi!
Materiale necesare:
Pahar Erlenmeyer cu apă distilată, dop de cauciuc prevăzut cu termometru, sită de azbest, spirtieră, chibrit, ceas sau
cronometru.
Descrierea experimentului:
- Închide apa din pahar cu ajutorul dopului prevăzut cu termometru astfel încât termometrul să nu intre în apă și încălzește apa prin intermediul sitei la flacăra spirtierei.
- Măsoară timpul de la începerea încălzirii și completează următorul tabel:

Concluzia experimentului:
- Apa are proprietatea de a fierbe la 100 °C – proprietate fizică, fiindcă nu schimbă compoziția substanței (aburul este tot apă, dar în stare gazoasă)
- Fiecare lichid începe să fiarbă la o anumită temperatură, numită temperatură de fierbere, care este o constantă de material (vezi tabel cu constante de la sfârșitul manualului). Pe parcursul fierberii temperatura de fierbere este constantă.
I.6.4. Aplică ce ai învățat la Proprietăţi fizice şi chimice.
📝 Temă
1. Precizează felul proprietății: fizică sau chimică.
A. Piatra vânătă este solubilă în apă.
B. Lemnul are proprietatea de a putrezi.
C. Hârtia are proprietatea de a arde.
D. Fierul are proprietatea de a fi atras de magnet.
E. Dioxidul de carbon este un gaz incolor.
F. Cuprul are proprietatea de a cocli.
G. Sulful este izolator termic și electric.
H. Mercurul este lichid.
I. Ceața se formează prin condensarea vaporilor de apă din aer la suprafața pământului.
J. Gheața se topește în palmele copilului.