II.1. Pure substances and mixtures of substances
Pure substance has a well-defined composition, has specific physical constants and retains its composition following physical phenomena.
Required Materials:
2 glasses of Berzelius, water, glass rod, extra fine salt and coarse salt.
Experiment description:
- Put the extra fine salt in one glass and the coarse salt in the other.
- Add water to both glasses and dissolve the salt by stirring with the wand and then observe the contents of the 2 glasses.
- What do you notice?
In the glass with water and fine salt, the components are indistinguishable, and in the other, dark substances are deposited at the bottom.
Experiment conclusion:
Fine salt is a pure substance, and coarse salt is impure, that is, it contains impurities (other substances besides salt).
To express how clean a substance is, the notion of purity is used.
Purity (p) represents the mass of pure substance found in 100 g of impure substance. It is expressed in percentage (%).
1. 700 g of pure salt is obtained from 800 g of coarse salt. Calculate the purity of the deposit and the percentage of impurities.
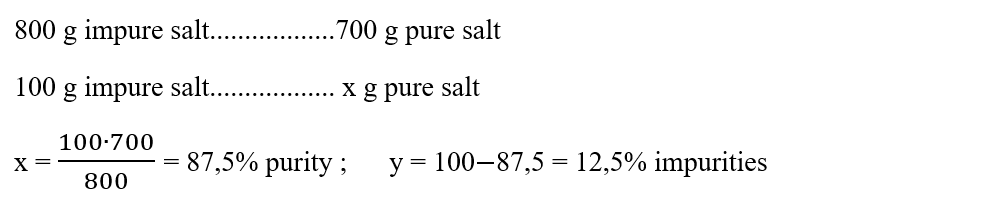
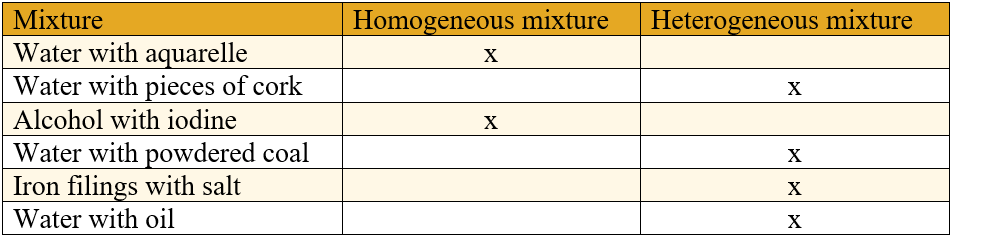
Mixture is the result of combining two or more substances between which no chemical phenomena (chemical reactions) occur.
Every day you use mixtures in different forms: culinary preparations, medicines, toothpaste, detergents, cosmetics, etc.
🔥 Warning! This experiment is for teachers only!
🔥 Warning! Mercury is extremely toxic! Do not inhale its fumes! Don't touch it or taste it!
Required Materials:
Mortar with pestle, sulphur, iron filings, a drop of mercury, magnet.
Experiment description:
- Put sulfur powder and iron filings in a mortar and mix with the pestle.
- Near this mixture a magnet.
- What do you notice?
The iron in the mixture does not lose its property of being attracted to the magnet.
Sulfur in the mixture also retains its properties (yellow powder).
- Put sulfur powder and a drop of mercury in a mortar and mix with the pestle.
- What do you notice?
In a short time you will notice a black powder and the formation of a new substance that no longer has the properties of the 2 components.
Experiment conclusion:
Sulfur and iron mixed in a mortar form a mixture.
Sulfur with mercury undergoes a chemical phenomenon, as a result of which the two substances no longer retain their properties.
Characteristics of the mixtures
A) A mixture consists of two or more components.
B) The component substances can be taken in different proportions.
C) Each component substance retains its physical and chemical properties.
A mixture can consist of:
- Solid substances: soil, rocks, alloys
- Liquid substances: various drinks, antifreeze
- Gaseous substances: air, natural gases
- Substances in the 3 states of aggregation: cloudy water (water + solid particles + dissolved air)
Obtaining of the mixtures:
a) The mixing of the components in the gaseous state takes place by itself through the phenomenon of diffusion, due to the disordered and continuous movement of the component particles.
b) Mixing a liquid with:
- a gas by bubbling (blowing) the gas into the liquid;
- another liquid, also by itself through diffusion;
- a solid, by mixing and dissolving.
c) Mixing the components in a solid state using a pestle and mortar.
According to their composition, the mixtures are classified into:
1. Homogeneous mixtures that have the same composition and properties throughout their mass.
Examples of homogeneous mixtures:
- solutions;
- alloys;
- vinegar;
- air;
- spirit

2. Heterogeneous mixtures that do not have the same composition and the same properties throughout their mass and its components are visible with the naked eye or with a magnifying glass.
Examples of heterogeneous mixtures:
- water with oil;
- water with sand;
- air with mercury;
- food dishes;
- rocks etc.

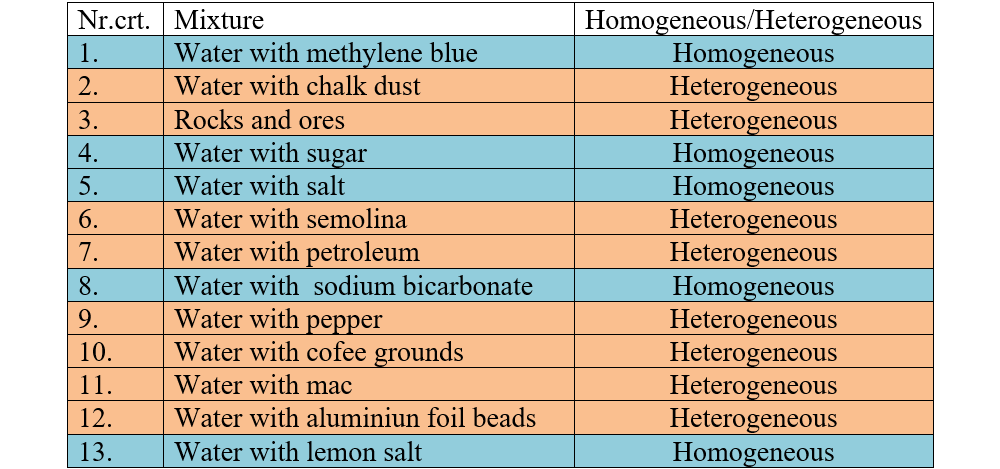
Required Materials:
Aluminum foil beads, chalk dust, semolina, ink (methylene blue), lemon salt, pepper, petroleum,
coffee grounds, sodium bicarbonate, transparent glasses.
Experiment description:
- Put a little water (10 mL) in each glass. Add to each the different substances you have in the house (shown to necessary materials or others). Carefully mix the contents of each glass.
- Analyze the appearance of each mixture and observe with the naked eye if they have the same composition and the same properties in all their mass.
- Complete the following table to write what kind of mixture each is.
Experiment conclusion:
If one substance dissolves in another substance, a solution is formed, which is a homogeneous mixture.
The homogeneous mixture has the same composition and the same properties throughout its mass.
The heterogeneous mixture does not have the same composition and the same properties throughout its mass (it can be
seen with the naked eye or with the microscope of its components).
1. Give 3 examples of pure substances, homogeneous and heterogeneous mixtures.

2. Identifies the mixture type from the table.