II.2.2. Methods of separating the components of a heterogeneous mixture
II.2.2.1. Decantation
Decantation is the method of separating the components of a heterogeneous solid-liquid or liquid-liquid mixture based on the large difference between their densities.
a) For solid-liquid components with much different densities, decantation with the aid of a wand is used (when the solid sits at the bottom of the liquid).
b) For liquid-liquid components with different densities and which are immiscible (they do not dissolve in each other), decantation with a separatory funnel is used.
Required Materials:
2 Berzelius glasses, wand, separating funnel (with tap), water, oil, sand, stand.
Experiment description:
- Using the wand, pour the liquid part of the mixture into the separatory funnel. The sand remains in the glass.
- Open the valve of the separatory funnel so that the water with the higher density will flow into the glass, and the oil with the lower density will remain in the separatory funnel.
- What do you notice?
By decantation, I separated the sand from the water with oil, with a stick, then I separated the water from the oil with a funnel with a tap.
Experiment conclusion:
By decantation, we separated the components of a heterogeneous solid-liquid or liquid-liquid mixture, based on the difference between their densities.
1. Obtaining potable water in large decantation basins, where solid impurities settle to the bottom of the decantation basins and the water is clarified and purified (cleaned) mechanically.

2. Separation of the 2 components of slaked lime: lime water and milk of lime.

II.2.2.2. Filtration
Filtration is the method of separating a solid substance from a heterogeneous solid-liquid mixture that have close densities, with the help of a filter material (filter paper).
When the density of the solid is much higher than that of the liquid in the mixture, we apply decantation, and when the density of the solid is similar to that of the liquid (the solid does not settle at the bottom of the liquid, but is scattered throughout the liquid) we apply filtration.
Required Materials:
1 Berzelius beaker, 1 Erlenmeyer beaker, rod, funnel, filter paper, water, sulfur powder (coal powder, chalk dust, cork grains, tea leaves, ground coffee, etc.).
Experiment description:
- Mix the water with the sulfur powder in the Berzelius glass.
- Fold the filter paper in four, separate one sheet from the others, apply it to the inner walls of the moistened funnel.
- The mixture is poured into the funnel on a glass rod inclined towards the side wall, towards the triple side of the filter.
- What do you notice?
I used filtration to separate the water from the sulfur powder.
Experiment conclusion:
Through filtration, I separated a solid substance from a heterogeneous solid-liquid mixture, with similar densities, with the help of a filter paper.
1. Drinking water filter (good for drinking).

2. Air filter (automotive, air conditioning, vacuum cleaners, industrial for the retention of polluting gases, etc.)

3. Automotive oil filter.

4. Coffee filter.

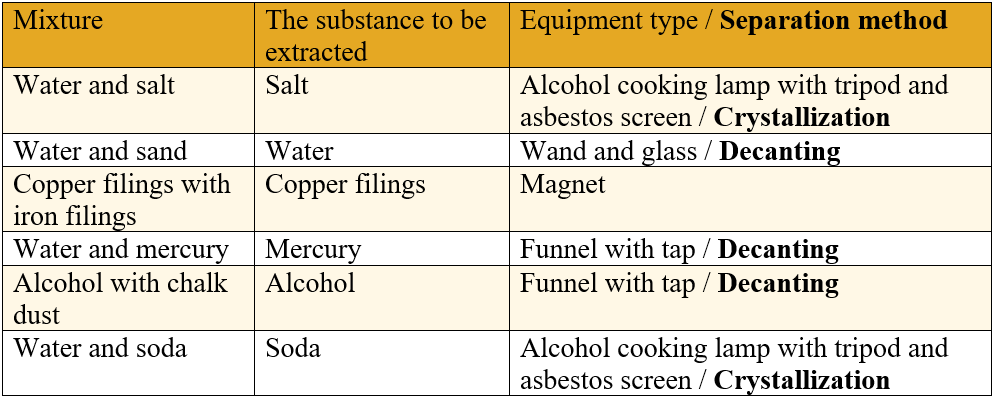
1. Tudor has the following tools at his disposal: Alcohol cooking lamp with tripod and asbestos sieve, magnet, funnel with filter, glasses, wand, funnel with tap.
What equipment is needed to extract each substance listed in column 2 (Substance to be extracted)? Write the answers in the table.